CiPA on Drug Discovery, Development, and Regulatory Pathways: Insights from David Strauss (FDA)
Archival Context: This article reconstructs the key insights from the presentation delivered by David Strauss, M.D., Ph.D. (FDA), at the Cardiac Safety Research Consortium (CSRC) meeting on May 20, 2018. As cited in the MDPI Journal of International Molecular Sciences, this presentation outlined the regulatory shift from the traditional hERG-centric approach to the mechanistic “Comprehensive in vitro Proarrhythmia Assay (CiPA)” initiative.
Executive Summary
For years, drug safety relied on the “Thorough QT” (TQT) study paradigm, which focused almost exclusively on blocking the hERG potassium channel. While successful in preventing torsadogenic drugs from reaching the market, this approach was prone to “false positives”—discarding potentially beneficial drugs that blocked hERG but were not actually proarrhythmic.
In his 2018 address, Dr. David Strauss detailed the potential role of CiPA in reshaping drug discovery. By integrating in vitro ion channel assessment, in silico modeling, and stem cell assays, this new pathway allows developers to “design out” cardiotoxicity early in the pipeline using advanced chemical biology tools.
Part I: The Limitations of the Single-Channel Approach
The traditional safety paradigm assumed that hERG block equals arrhythmia risk. Dr. Strauss explained that this is an oversimplification. Many drugs act as “Balanced Ion Channel Blockers.”
- Example: A drug might block hERG (promoting arrhythmia) but also block Late Sodium ($I_{NaL}$) or Calcium ($I_{CaL}$) channels (preventing arrhythmia).
- Result: The net effect is neutral, but under the old guidelines, the drug would be killed due to the hERG signal.
Part II: The CiPA Pillars – A Demand for Multi-Channel Screening
The CiPA framework introduces a requirement for comprehensive profiling against a panel of cardiac ion channels. This shifts the burden from clinical monitoring to pre-clinical screening.
1. In Vitro Assessment of Multiple Ion Channels
CiPA mandates characterization of the drug’s effect on:hERG,Cav1.2,Nav1.5 and KCNQ1
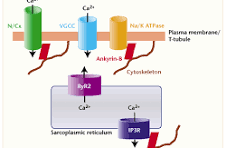
- Figure 1. The CiPA panel requires screening against multiple ion channels on the cardiomyocyte membrane. High-purity ion channel inhibitors are used as reference standards to validate these assays.
Implication for Discovery: To generate regulatory-grade data, laboratories must validate their patch-clamp assays using specific reference compounds. Access to high-purity selective inhibitors (e.g., Dofetilide for hERG, Verapamil for Cav1.2, Mexiletine for Nav1.5) is critical for calibrating these systems.
2. In Silico Modeling
Experimental data is fed into a computational model of the adult human ventricular cardiomyocyte. This model integrates the opposing effects of blocking multiple channels to predict the net risk of Early Afterdepolarizations (EADs).
3. Human Stem Cell-Derived Cardiomyocytes (hiPSC-CMs)
Confirmatory testing is performed in hiPSC-CMs.
- Tooling: The differentiation and maturation of these cells require precise chemical modulation using small molecule inhibitors (e.g., GSK3 inhibitors) to ensure an adult-like phenotype.
Part III: Implementing CiPA in Lead Optimization
Dr. Strauss emphasized that CiPA is a tool for Drug Discovery, not just regulation. By implementing CiPA protocols early, companies can select lead candidates with the best safety profiles.
High-Throughput Screening (HTS)
Modern medicinal chemistry programs now run parallel screens:
- Therapeutic Potency: Does it hit the target?
- CiPA Liability: Does it hit the ion panel?
To support this, researchers utilize annotated compound libraries containing known cardiotoxins (positive controls) and non-toxins (negative controls) to train their internal assays.
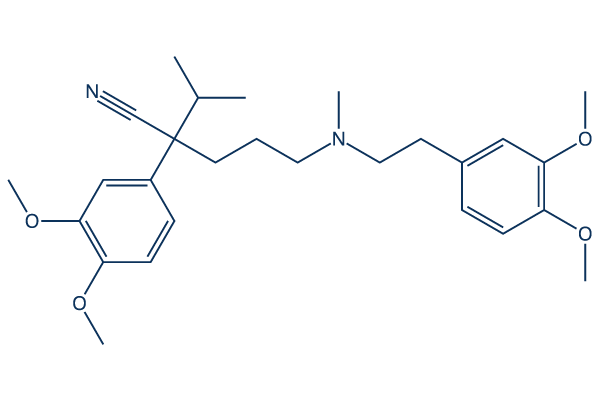
- Figure 2. Chemical structure of Verapamil. A classic calcium channel blocker, Verapamil serves as a crucial reference compound in CiPA studies to demonstrate how multi-channel blocking can mitigate Torsades de Pointes risk.
Part IV: Regulatory Pathways and Future Directions
The 2018 presentation concluded with a roadmap for regulatory acceptance. As the validation of the CiPA model progresses, the FDA increasingly accepts mechanistic data to waive TQT studies.
Conclusion for Developers:
Success in the CiPA era requires a robust toolkit. From reference inhibitors for assay validation to chemical probes for determining mechanism of action, the quality of the chemical reagents used in early discovery directly correlates with the reliability of the safety dossier submitted to the FDA.
References
- Strauss, D. “CiPA on Drug Discovery, Development, and Regulatory Pathways.” CSRC Meeting Presentation, May 2018.
- Park, M. et al. QT Assessment in Early Drug Development: The Long and the Short of It. Int. J. Mol. Sci. 20, 1324 (2019).
- Colatsky, T. et al. The Comprehensive in Vitro Proarrhythmia Assay (CiPA) initiative. J Pharmacol Toxicol Methods 81, 15-20 (2016).
